|
Combination Lipid-altering Drug Therapy with Statins: An Update


|
|


Statins are currently the most prescribed lipid-altering drugs because
of their efficacy in favorably altering blood lipid levels, safety and
tolerability, and proven benefits on reducing atherosclerotic coronary
heart disease (CHD) events. Combination lipid-altering drug therapy
with statins is often indicated for patients: (1) who are unable to
achieve recommended treatment goals with statin monotherapy, (2) who
may be at risk for intolerance, toxicity, or adverse drug interactions
with higher-dose statin monotherapy, or (3) who may benefit from the
use of one or more lipid-altering drugs in combination with statins
resulting in complementary benefits towards further reduction in CHD
risk. Some of the lipid-altering agents most commonly used in
combination with statins include ezetimibe, bile acid sequestrants,
peroxisome proliferator-activated receptor (PPAR) agonists, fish oils,
and niacin. A potentially important investigational lipid-altering drug
combination is statin in combination with a cholesteryl ester transport
inhibitor.
|
|


The National Cholesterol Education Program Adult Treatment Panel III
(ATP III) defines high-risk patients as those who have CHD or
atherosclerotic disease of the blood vessels to the brain or
extremities, or diabetes, or multiple (2 or more) risk factors (e.g.,
smoking, hypertension) that give them a >20% chance of having a
heart attack within 10 years. Very high risk patients are those who
have cardiovascular disease together with (1) multiple risk factors
(especially diabetes), or severe and poorly controlled risk factors
(e.g., continued smoking), (2) metabolic syndrome (a constellation of
risk factors associated with obesity including high triglycerides and
low high-density lipoprotein cholesterol [HDL-C]) and/or (3) acute
coronary syndromes such as heart attack. For high-risk patients, the
overall goal is to achieve a low-density lipoprotein cholesterol
(LDL-C) level of <100 mg/dL. But for patients at very high risk, a
group that is considered a "subset" of the high-risk category, a
therapeutic option is to decrease LDL-C levels to <70 mg/dL. For
very high risk patients whose LDL-C levels are already <100 mg/dL,
there is also an option to use drug therapy to reach the <70-mg/dL
treatment goal.
For moderately high risk patients, the goal is to achieve an
LDL-C level <130 mg/dL, but it is a therapeutic option to set a
lower LDL-C goal of <100 mg/dL and to use drug therapy for LDL-C
levels of 100–129 mg/dL to achieve this lower goal. For high-risk or
moderately high risk patients, the report advises that LDL-C–lowering
drug therapy be sufficient to achieve at least a 30–40% reduction in
LDL-C levels. This can be accomplished by taking statins or by
combining lower doses of statins with other drugs (bile acid resins,
nicotinic acid, or ezetimibe) or with food products containing plant
stanols/sterols. For moderately high risk patients with LDL-C levels of
100–129 mg/dL at baseline or on lifestyle therapy, LDL-C–lowering
therapy to reach a goal of <100 mg/dL is recommended, and
lipid-altering drug therapy to achieve this goal is a therapeutic
option. By definition, almost all people with 0–1 risk factor have a
10-year risk <10%. Drug therapy for primary prevention in this
patient population is recommended only for those with higher LDL-C
levels. Major CHD risk factors include cigarette smoking, blood
pressure >140/90
mm Hg or on antihypertensive medication, HDL-C <40 mg/dL (1.0
mmol/L), family history of premature CHD (CHD in male first-degree
relative aged <55 years; CHD in female first-degree relative aged
<65 years), and age (men >45 years; women >55 years). HDL-C >60 mg/dL (>1.6
mmol/L) counts as a "negative" risk factor; its presence removes 1 risk
factor from the total count. Framingham risk score may also be used to
determine 10-year CHD risk.

- National
Cholesterol Education Program (NCEP) Expert Panel on Detection,
Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult
Treatment Panel III). Third Report of the National Cholesterol
Education Program (NCEP) Expert Panel on Detection, Evaluation, and
Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel
III) final report. Circulation 2002;106:3143-3421.
- Grundy
SM, Cleeman JI, Bairey Merz CN, et al., for the Coordinating Committee
of the National Cholesterol Education Program. Implications of recent
clinical trials for the National Cholesterol Education Program Adult
Treatment Panel III guidelines. Circulation 2004;110:227-239.
- Bays
H, Shepherd J. Diabetes, metabolic syndrome and dyslipidemia. In:
Management Strategies in Diabetes. Hackensack, NJ: Cambridge Medical
Publications, 2004:1-28.
|
|


Over the years, various surveys have documented that the very patients
who are at highest CHD risk appear to be the least likely to achieve
lipid treatment goals. One such survey was the Lipid Treatment
Assessment Project (L-TAP). This analysis evaluated 4,888 patients
(4,137 treated with drug therapy and 751 treated with diet and
exercise) from 5 regions of the United States who were being treated
for hypercholesterolemia in a primary care setting from August 1996 to
February 1997. Of these patients, 30% had established CHD, 47% had >2
risk factors without evidence of CHD (high-risk group), and 23% had
<2 risk factors (low-risk group). Although current achievement of
lipid treatment goals may be improved over what may have been achieved
in 1996–1997 (based upon this L-TAP analysis), it continues to be true
that many of those patients at highest CHD risk often do not achieve
lipid treatment goals.

- Pearson
TA, Laurora I, Chu H, Kafonek S. The Lipid Treatment Assessment Project
(L-TAP): a multicenter survey to evaluate the percentages of
dyslipidemic patients receiving lipid-lowering therapy and achieving
low-density lipoprotein cholesterol goals. Arch Intern Med
2000;160:459-467.
|
|


Many potential reasons exist as to why patients do not achieve lipid
treatment goals. For example, some patients are never treated with any
lipid-altering drug. Patient noncompliance may also be a factor. Known,
potential, or perceived issues of tolerability and safety of high-dose
statins among clinicians and patients may pose therapeutic challenges.
However, the resistance among many clinicians to use the
highest doses of statins alone is due to not only concerns about
toxicity and intolerability (which most often occur at higher doses),
but also the recognition that most LDL-C reduction with statins occurs
at the lower doses. In fact, each doubling of the statin dose produces
an average additional decrease in LDL-C levels of about 5–6%, based
upon the baseline LDL-C value ("rule of 6"). In the example above,
atorvastatin 10 mg/day lowered mean LDL-C levels by 38%. Upon titration
to 20 mg, 40 mg, and 80 mg, LDL-C levels were lowered only an
additional 8%, 5%, and 3% respectively (based upon the baseline,
pretreatment LDL-C level) for a total further reduction of 16% with
these three titrations. In other words, starting with a mean baseline
LDL-C level of 211 mg/dL, atorvastatin 10 mg/day would be expected to
lower LDL-C levels to about 131 mg/dL (38% reduction). A three-step
doubling of the dose to atorvastatin 80 mg/day would lower the baseline
mean LDL-C levels an additional 16% to about 97 mg/dL (38% + 16% = 54%
total reduction). The modest percent reduction after the lowest doses
is also seen with the above simvastatin example, in which simvastatin
10 mg lowered mean LDL-C levels by 28%, while titration to 20 mg and 40
mg resulted in only an additional 7% and 6% reduction in LDL-C compared
with the baseline value.
Finally, many patients with more severe dyslipidemias and/or
in need of the most aggressive lipid therapy may not achieve lipid
treatment goals even when the highest dose of statin is used. For all
of these reasons, combination lipid-altering drug therapy is often
indicated to avoid known or potential toxicity with higher doses of
statin alone, or when statin monotherapy is insufficiently effective at
the higher doses.

- Illingworth DR. Management of hypercholesterolemia. Med Clin North Am 2000;84:23-42.
- Bays H. Ezetimibe. Expert Opin Investig Drugs 2002;11:1587-1604.
- Jones
P, Kafonek S, Laurora I, Hunninghake D, for the CURVES Investigators.
Comparative dose efficacy study of atorvastatin versus simvastatin,
pravastatin, lovastatin, and fluvastatin in patients with
hypercholesterolemia (the CURVES study). Am J Cardiol 1998;81:582-587.
|
|


Conceptually, it might be argued that circulating cholesterol
originates from predominantly two sources: synthesis (from liver and
peripheral tissues) and absorption (from the intestine). Irrespective
of the origins of cholesterol, it is the liver that normally serves as
the main regulatory organ that determines LDL-C blood levels.
(Cholesterol from endothelial macrophages associated with arterial
cholesterol plaques are clinically important, but only a very minor
contributor to total circulating cholesterol.)
Animal data suggest that most cholesterol synthesis in the
body is from peripheral tissues such as intestine, muscle, and skin.
The greatest amount of cholesterol produced per gram of tissue is from
endocrine organs such as the ovary, adrenal glands, and
gastrointestinal tract. This is because cholesterol is the "backbone"
precursor for many hormones.
The relative contribution of cholesterol from any of these sources is
dependent upon genetic predisposition, diet, drug therapies, interplay
of enzymatic up- and down-regulations, and other potential factors.
Decreased cholesterol contribution to the liver may increase hepatic
LDL receptor activity and thus reduce circulating LDL-C blood levels,
which in turn is associated with reduced risk for CHD. Thus, different
lipid-altering drugs whose mechanism of action reduces different
sources of cholesterol may have complementary actions in lowering
LDL-C.
Additional abbreviation on slide: SR-B1 = scavenger receptor class B, type 1.

- Bays H, Dujovne C. Colesevelam HCl: a non-systemic lipid-altering drug. Expert Opin Pharmacother 2003;4:779-790.
- Dietschy
JM, Turley SD, Spady DK. Role of liver in the maintenance of
cholesterol and low density lipoprotein homeostasis in different animal
species, including humans. J Lipid Res 1993;34:1637-1659.
- Spady
DK, Dietschy JM. Sterol synthesis in vivo in 18 tissues of the squirrel
monkey, guinea pig, rabbit, hamster, and rat. J Lipid Res
1983;24:303-315.
|
|
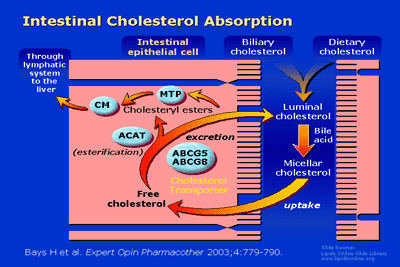

Intestinal cholesterol absorption is an important origin of circulating
LDL-C. Although dietary cholesterol does contribute, the majority (2/3
to 3/4) of cholesterol delivered to the intestine is derived from
biliary cholesterol excretion. Intestinal cholesterol undergoes
micellar adaptation by bile acids and is then absorbed into the
intestinal cells. The ensuing free cholesterol may subsequently be
"pumped" back into the intestine through adenosine triphosphate–binding
cassette (ABC) transporters ABCG5 and ABCG8. Alternatively, intestinal
free cholesterol may be esterified through acyl-coenzyme A:cholesterol
acyltransferase (ACAT), and then packaged into chylomicrons (CMs) in
the intestinal epithelial cell by microsomal triglyceride transfer
protein (MTP). As CMs leave the intestine, their cholesterol is
transported through the lymphatic system to the liver.

- Bays H, Dujovne C. Colesevelam HCl: a non-systemic lipid-altering drug. Expert Opin Pharmacother 2003;4:779-790.
- Bays H. Ezetimibe. Expert Opin Investig Drugs 2002;11:1587-1604.
|
|


Ezetimibe is the first in the class of cholesterol absorption
inhibitors, whose mechanism of action is consistent with the binding to
and blockade of a sterol transporter on the brush border membrane of
intestinal epithelial cells. Radiolabeled ezetimibe is clearly found on
the brush border where a transporter would be expected to reside. While
a specific cholesterol transporter remained elusive for years, a
Niemann-Pick C1 Like 1 Protein transporter has been discovered that
appears to be critical for intestinal cholesterol absorption.
Through inhibition of intestinal cholesterol absorption,
ezetimibe effectively reduces the amount of biliary/dietary cholesterol
delivered to the liver (via chylomicrons and chylomicron remnants) and
reduces the cholesterol content of atherogenic particles
(chylomicrons/chylomicron remnants, VLDL, LDL). The reduced delivery of
intestinal cholesterol to the liver increases hepatic LDL receptor
activity and increases clearance of circulating LDL-C. LDL-C levels and
LDL particles are thereby reduced.

- Catapano AL. Ezetimibe: a selective inhibitor of cholesterol absorption. Eur Heart J Suppl 2001;3:E6-E10.
- Bays H. Ezetimibe. Expert Opin Investig Drugs 2002;11:1587-1604.
- Altmann
SW, Davis HR Jr, Zhu LJ, et al. Niemann-Pick C1 Like 1 protein is
critical for intestinal cholesterol absorption. Science
2004;303:1201-1204.
|
|


The pharmacokinetic properties of ezetimibe make it suitable for
once-daily administration. The time to maximum ezetimibe concentration
is 4–12 hours.

- Zaks
A, Dodds DR. Enzymatic glucuronidation of a novel cholesterol
absorption inhibitor, SCH 58235. Appl Biochem Biotechnol
1998;73:205-214.
- Bays H. Ezetimibe. Expert Opin Investig Drugs 2002;11:1587-1604.
- van
Heek M, Farley C, Compton DS, et al. Comparison of the activity and
disposition of the novel cholesterol absorption inhibitor, SCH58235,
and its glucuronide, SCH60663. Br J Pharmacol 2000;129:1748-1754.
|
|


In two 12-week studies, ezetimibe monotherapy significantly decreased
plasma LDL-C levels and increased plasma HDL-C levels, with a
tolerability profile similar to that of placebo. The 10-mg dose of
ezetimibe provided greater benefit on blood LDL-C level reduction
compared with 5 mg, without affecting tolerability and without any
significant increase in adverse events. Therefore, the 10-mg dose of
ezetimibe was chosen as the only dose for phase III clinical trials.
The 10-mg dose of ezetimibe was subsequently submitted to the FDA for
approval in December 2001 and was approved for clinical use in November
2002.

- Bays
HE, Moore PB, Drehobl MA, et al., for the Ezetimibe Study Group.
Effectiveness and tolerability of ezetimibe in patients with primary
hypercholesterolemia: pooled analysis of two phase II studies. Clin
Ther 2001;23:1209-1230.
|
|


In this practical and clinically relevant randomized placebo-controlled
trial, the efficacy and safety of ezetimibe "added on" to ongoing
statin therapy was evaluated in 769 patients with hypercholesterolemia,
CHD, and/or multiple risk factors. All subjects continued on stable
doses of whatever statin had been prescribed by their treating
clinician. They were eligible to enter the study if they had not
achieved their NCEP ATP II LDL-C treatment goal with statin alone.
After a 6-week run-in, about half of subjects were randomized to
ezetimibe 10 mg once a day plus their statin, and the other half were
randomized to placebo plus their statin. The primary endpoint was the
percent LDL-C reduction at 8 weeks.

- Gagné
C, Bays HE, Weiss SR, et al, for the Ezetimibe Study Group. Efficacy
and safety of ezetimibe added to ongoing statin therapy for treatment
of patients with primary hypercholesterolemia. Am J Cardiol
2002;90:1084-1091.
|
|


After 8 weeks in the "Add on" study, the addition of ezetimibe to
ongoing statin therapy significantly reduced LDL-C, increased HDL-C,
and decreased triglyceride levels compared with placebo plus statin.

- Gagné
C, Bays HE, Weiss SR, et al, for the Ezetimibe Study Group. Efficacy
and safety of ezetimibe added to ongoing statin therapy for treatment
of patients with primary hypercholesterolemia. Am J Cardiol
2002;90:1084-1091.
|
|


Ezetimibe added to stable statin dose was markedly superior to placebo
added to stable statin dose in achievement of NCEP ATP II LDL-C
treatment goals.

- Gagné
C, Bays HE, Weiss SR, et al, for the Ezetimibe Study Group. Efficacy
and safety of ezetimibe added to ongoing statin therapy for treatment
of patients with primary hypercholesterolemia. Am J Cardiol
2002;90:1084-1091.
|
|


In a study of 628 patients with primary hypercholesterolemia, ezetimibe
plus 10 mg of atorvastatin was more effective than atorvastatin 10 mg,
20 mg, or 40 mg in lowering LDL-C levels. In fact, the LDL-C–lowering
efficacy of 10 mg of ezetimibe plus 10 mg of atorvastatin was similar
to 80 mg of atorvastatin. One method used to remember this clinical
finding is the "formula" of "10 + 10 = 80."

- Ballantyne
CM, Houri J, Notarbartolo A, et al., for the Ezetimibe Study Group.
Effect of ezetimibe coadministered with atorvastatin in 628 patients
with primary hypercholesterolemia: a prospective, randomized,
double-blind trial. Circulation 2003;107;2409-2415.
|
|


Three hours after intravenous administration to rats, the majority of
radioactively labeled ezetimibe localized to the intestinal lumen and
some of it was associated with the intestinal wall. In contrast, minute
ezetimibe-derived radioactivity was found in plasma, liver, or bile.
Therefore, although ezetimibe is technically a "systemic" drug because
of its enterohepatic circulation and recirculation, the low systemic
exposure of ezetimibe may help reduce its potential for systemic
adverse effects and for drug interactions.

- van
Heek M, Farley C, Compton DS, et al. Comparison of the activity and
disposition of the novel cholesterol absorption inhibitor, SCH58235,
and its glucuronide, SCH60663. Br J Pharmacol 2000;129:1748-1754.
|
|


At least in part because of its limited systemic exposure, ezetimibe
has limited drug interactions. Although colesevelam may have less
potential for adverse drug interactions with ezetimibe than
cholestyramine, the potential efficacy and drug interactions of the
combination of colesevelam and ezetimibe have not been evaluated and/or
reported. Mild increases in ezetimibe (less than a factor of 2) have
been found with concomitant fibrate administration (gemfibrozil and
fenofibrate). However, information on the safety and effectiveness of
ezetimibe and fibrates awaits the results of ongoing studies.

- Kosoglou
T, Meyer I, Veltri EP, et al. Pharmacodynamic interaction between the
new selective cholesterol absorption inhibitor ezetimibe and
simvastatin. Br J Clin Pharmacol 2002;54:309-319.
- Bays H. Ezetimibe. Expert Opin Investig Drugs 2002;11:1587-1604.
- Kosoglou
T, Guillaume M, Sun S, et al. Pharmacodynamic interaction between
fenofibrate and the cholesterol absorption inhibitor ezetimibe
[abstract]. Atheroscler Suppl 2001;2:38.
- Zhu
Y, Statkevich P, Kosoglou T, et al. Effect of SCH 58235 on the activity
of drug metabolizing enzymes in vivo [abstract]. Clin Pharmacol Ther
2000;67:152.
- Stein E. Results
of phase I/II clinical trials with exetimibe, a novel selective
cholesterol absorption inhibitor. Eur Heart J Suppl 2001;3:E11-E16.
|
|


Ezetimibe is indicated for use as monotherapy and combination therapy with statins in patients with hypercholesterolemia.

- Bays H. Ezetimibe. Expert Opin Investig Drugs 2002;11:1587-1604.
- Dujovne
CA, Ettinger MP, McNeer JF, et al. Efficacy and safety of a potent new
selective cholesterol absorption inhibitor, ezetimibe, in patients with
primary hypercholesterolemia. Am J Cardiol 2002;90:1092-1097.
- Gagné
C, Bays HE, Weiss SR, et al, for the Ezetimibe Study Group. Efficacy
and safety of ezetimibe added to ongoing statin therapy for treatment
of patients with primary hypercholesterolemia. Am J Cardiol
2002;90:1084-1091.
- Gagné
C, Gaudet D, Bruckert E, et al. Efficacy and safety of ezetimibe
coadministered with atorvastatin or simvastatin in patients with
homozygous familial hypercholesterolemia. Circulation
2002;105:2469-2475.
- Salen
G, von Bergmann K, Kweiterovich P, et al. Ezetimibe is an effective
treatment for homozygous sitosterolemia [abstract]. Circulation
2002;106:II-185.
|
|


Ezetimibe is also indicated for treatment of homozygous sitosterolemia,
a very rare inherited genetic disorder of the ABCG5/ABCG8 transporter
protein. Phytosterols (such as sitosterol and campesterol) are not
synthesized in humans and are derived entirely from the diet. Once
absorbed by intestinal cells, ABCG5/ABCG8 transporters normally pump
these potentially atherogenic phytosterols back into the intestinal
lumen, normally resulting in <5% net absorption. Dysfunctional
ABCG5/ABCG8 sterol transporters result in a net hyperabsorption of
phytosterols, leading to tendinous and tuberous xanthomas and premature
atherosclerosis that may or may not be accompanied by elevations in
blood cholesterol levels. Ezetimibe inhibits net phytosterol absorption
in these patients and is the only drug with an indication for treatment
of homozygous sitosterolemia.
ABC transporters function to translocate or "pump" various
compounds (such as sugars, amino acids, metal ions, peptides, proteins,
and a large number of hydrophobic compounds and metabolites) across the
membranes of cells and tissues. The ABC transporter superfamily is the
largest transporter gene family and is typically classified into
subfamilies of A–G.
Tangier disease is an inherited recessive disorder resulting
from a defect in the ABCA1 transporter, which is responsible for the
transport of cholesterol from peripheral tissues to HDL particles. No
normal HDL exists in this disorder, and patients have very low levels
of an abnormal HDL variant. Clinically, patients have yellow-orange
tonsils, peripheral neuropathy, and hepatosplenomegaly thought to be
related to the accumulation of cholesteryl esters in body tissues and
macrophages.
Sitosterolemia is an inherited autosomal recessive disorder
resulting from a defect in enterocyte ABCG5 and/or ABCG8, responsible
for the transport of intestinally absorbed cholesterol and plant
sterols/stanols back to the intestinal lumen. Clinically, patients
express tendinous and tuberous xanthomas and premature atherosclerosis
without necessarily having elevations in blood cholesterol levels.

- Bodzioch
M, Orso E, Klucken J, et al. The gene encoding ATP-binding cassette
transporter 1 is mutated in Tangier disease. Nat Genet 1999;22:347-351.
- Bays
HE, Stein EA. Pharmacotherapy for dyslipidemia: current therapies and
future agents. Expert Opin Pharmacother 2003;4:1901-1938.
- Rust
S, Rosier M, Funke H, et al. Tangier disease is caused by mutations in
the gene encoding ATP-binding cassette transporter 1. Nat Genet
1999;22:352-355.
|
|


Ezetimibe is contraindicated in patients with moderate or severe
hepatic insufficiency. The combination of ezetimibe and statins is
contraindicated in patients with active liver disease or unexplained
persistent elevations in liver transaminases. When ezetimibe is
administered with a statin in a woman of child-bearing potential, it is
recommended that the prescribing clinician refer to the pregnancy
category and product labeling for the statin. However, since statins
are not indicated for use in pregnant women (pregnancy X category), the
combined use of ezetimibe with statins is also not indicated during
pregnancy. The use of ezetimibe alone is in the pregnancy C category,
as no adequate studies have demonstrated safety in pregnant women.
Thus, the use of ezetimibe alone in pregnant women should be used only
if the potential benefit justifies the risk to the fetus.

- Bays H. Ezetimibe. Expert Opin Investig Drugs 2002;11:1587-1604.
|
|


None of the individual trials of ezetimibe monotherapy have
demonstrated significant increases in liver enzymes compared with
placebo. In the clinical trials of combination therapy with ezetimibe
plus statin versus placebo plus statin, the incidence of consecutive
transaminase elevations 3 or more times the upper limit of normal was
<2% in either group. However, the incidence of such liver enzyme
elevations was slightly higher (1.3%) in the ezetimibe plus statin
group than the placebo plus statin group (0.4%). These liver enzyme
elevations were asymptomatic and often transient, with resolution on
discontinuation of therapy or sometimes with continued therapy. For
this reason, it is recommended that if ezetimibe is to be used in
combination with a statin, liver enzyme monitoring should occur at
initiation of therapy and then according to the recommendations for the
statin. Some clinicians have interpreted this to mean that with regard
to liver enzyme monitoring, the addition of ezetimibe to a statin might
be considered analogous to increasing the dose of the statin. Ezetimibe
is not associated with increased myalgias or myopathy.

- Bays H. Ezetimibe. Expert Opin Investig Drugs 2002;11:1587-1604.
|
|


As with the use of other lipid-altering drugs, the administration of
ezetimibe should occur with appropriate management of potential
secondary causes of dyslipidemia, such as diabetes mellitus,
hypothyroidism, certain types of liver and kidney diseases, and
drug-induced dyslipidemia as might occur with various steroids,
ethanol, protease inhibitors, isotretinoin, etc.

- Bays H. Ezetimibe. Expert Opin Investig Drugs 2002;11:1587-1604.
|
|


The combination of ezetimibe and simvastatin is available in a single
tablet (Vytorin™). The rationale behind this lipid-altering therapy is
to harness the dual inhibition of hepatic cholesterol synthesis (with
simvastatin) and intestinal cholesterol absorption (with ezetimibe). A
typical starting dose of 10 mg ezetimibe/20 mg simvastatin lowers LDL-C
levels by about 50% or more, although an optional starting dose of 10
mg ezetimibe/40 mg simvastatin is available for patients in need of
more immediate and greater LDL-C lowering. At the top dose of 10 mg/80
mg, LDL-C levels are reduced by about 60%. In the study described
above, the mean LDL-C at baseline was 178 mg/dL, and LDL-C levels were
lowered to 70 mg/dL, which is of potential therapeutic importance given
the aggressive LDL-C treatment goals recommended for patients at
highest CHD risk.

- Bays
HE, Ose L, Fraser N, et al. A multicenter, randomized, double-blind,
placebo-controlled, factorial design study to evaluate the
lipid-altering efficacy, safety, and tolerability profile of the
ezetimibe/simvastatin tablet compared with ezetimibe and simvastatin
monotherapy in patients with primary hypercholesterolemia. Clin Ther
2004;26:1758-1773.
|
|


The lipid-altering efficacy of the coadministration of ezetimibe and
simvastatin to bioequivalent doses of ezetimibe/simvastatin in a single
tablet has been studied and compared to atorvastatin. At relative
starting doses, 10 mg ezetimibe/20 mg simvastatin once per day has been
shown to lower LDL-C levels more than atorvastatin at its starting
doses of 10 mg or 20 mg once a day. At the highest doses, 10 mg
ezetimibe/80 mg simvastatin lowers LDL-C more than atorvastatin 80 mg
per day.

- Ballantyne
CM, Blazing MA, King TR, et al. Efficacy and safety of ezetimibe
co-administered with simvastatin compared with atorvastatin in adults
with hypercholesterolemia. Am J Cardiol 2004;93:1487-1494.
|
|


Ezetimibe/simvastatin also provided persistent and consistent
elevations in HDL-C levels from the starting dose of 10 mg/20 mg up to
the top dose of 10 mg/80 mg. In multiple other clinical trials of
atorvastatin, further increases in HDL-C levels typically do not occur
with titration to the higher doses of atorvastatin. In fact, many
clinical trials have shown an attenuated effect upon HDL-C raising with
the higher 80-mg dose of atorvastatin (although the clinical
significance of this is unknown). As the result of the increase of
HDL-C levels with increasing doses of ezetimibe/simvastatin, coupled
with the attenuated effects upon HDL-C levels with increasing doses of
atorvastatin, the greatest differences in HDL-C levels in this
comparative study was at the top dose of ezetimibe/simvastatin (10
mg/80 mg) versus the top dose of atorvastatin (80 mg).

- Ballantyne
CM, Blazing MA, King TR, et al. Efficacy and safety of ezetimibe
co-administered with simvastatin compared with atorvastatin in adults
with hypercholesterolemia. Am J Cardiol 2004;93:1487-1494.
|
|


The triglyceride-lowering effects of ezetimibe/simvastatin versus
atorvastatin was roughly the same at starting doses, and at the highest
doses of each.

- Ballantyne
CM, Blazing MA, King TR, et al. Efficacy and safety of ezetimibe
co-administered with simvastatin compared with atorvastatin in adults
with hypercholesterolemia. Am J Cardiol 2004;93:1487-1494.
|
|


Bile acid sequestrants (resins) were among the first lipid-altering
drugs to show an ability to reduce the risk for CHD. Unfortunately, the
clinical use of bile acid resins such as cholestyramine and colestipol
has been limited because of common gastrointestinal side effects and
potential drug interactions with commonly prescribed concomitant drugs.
Colesevelam is a unique bile acid sequestrant polymer that has been
shown to have favorable lipid effects, better tolerability, and fewer
potential drug interactions than other bile acid sequestrants. Because
it is considered to be truly a nonsystemic drug, colesevelam would not
be expected to add adverse systemic effects when used in combination
with statins.
The efficacy of colesevelam has been evaluated in combination
with statins. In comparison to what would be expected with statins
alone, the administration of 4 tablets of colesevelam with statins has
been shown to result in an approximate 8–12% further reduction in LDL-C
levels, with variable effects on HDL-C and triglyceride levels.
Similarly, the administration of 6 tablets of colesevelam with statins
has been shown to result in an approximate 10–16% further reduction in
LDL-C levels, favorable effects upon HDL-C levels, and mild to moderate
elevations in triglyceride levels.

- Bays H, Dujovne C. Colesevelam HCl: a non-systemic lipid-altering drug. Expert Opin Pharmacother 2003;4:779-790.
- Davidson
MH, Dicklin MR, Maki KC, Kleinpell RM. Colesevelam hydrochloride: a
non-absorbed, polymeric cholesterol-lowering agent. Expert Opin
Investig Drugs 2000;9:2663-2671.
- Davidson
MH, Toth P, Weiss S, et al. Low-dose combination therapy with
colesevelam hydrochloride and lovastatin effectively decreases
low-density lipoprotein cholesterol in patients with primary
hypercholesterolemia. Clin Cardiol 2001;24:467-474.
- Knapp
HH, Schrott H, Ma P, et al. Efficacy and safety of combination
simvastatin and colesevelam in patients with primary
hypercholesterolemia. Am J Med 2001;110:352-360.
- Hunninghake
D, Insull W Jr, Toth P, et al. Coadministration of colesevelam
hydrochloride with atorvastatin lowers LDL cholesterol additively.
Atherosclerosis 2001;158:407-416.
|
|


The total LDL-C lowering with 6 tablets of colesevelam plus 10 mg of
atorvastatin is roughly equivalent to what is achieved with 80 mg of
atorvastatin. This may have clinical implications for patients who are
unable to tolerate higher statin doses or who are at risk for toxicity
and drug interactions at higher statin doses.

- Bays H, Dujovne C. Colesevelam HCl: a non-systemic lipid-altering drug. Expert Opin Pharmacother 2003;4:779-790.
- Hunninghake
D, Insull W Jr, Toth P, et al. Coadministration of colesevelam
hydrochloride with atorvastatin lowers LDL cholesterol additively.
Atherosclerosis 2001;158:407-416.
|
|


Each doubling of the statin dose produces an incremental LDL-C
reduction of approximately 5–6% of the baseline LDL-C value, resulting
in a further LDL-C reduction of 15–18% with a three-step doubling of
the statin, compared with the LDL-C reduction achieved with the statin
starting dose. The addition of 6 tablets of colesevelam or the addition
of 10 mg of ezetimibe to 10 mg of a statin results in about the same
level of LDL-C lowering as 80 mg of the statin. The highest doses of
statins are most associated with adverse side effects.

- Bays H, Dujovne C. Colesevelam HCl: a non-systemic lipid-altering drug. Expert Opin Pharmacother 2003;4:779-790.
|
|


Peroxisome proliferator-activated receptors (PPARs) are
ligand-activated nuclear transcription factors that, once activated,
increase the transcription of target genes. Activation of PPARs occurs
through ligand agonists that typically result in the subsequent
formation of a heterodimer complex with the nuclear receptor RXR
(PPAR/RXR heterodimer), which in turn regulates gene transcription by
binding to specific response elements (peroxisome proliferator response
element) that promote the activity of target genes. The ligands for the
PPAR-α receptor include fatty acids and fibrates. Fibrates have been
shown to reduce CHD in both primary and secondary prevention
monotherapy clinical trials. The ligands for the PPAR-γ receptor
include thiazolidinediones (TZDs), which have been shown to improve
glucose control.
Additional abbreviations on slide: BSEP = bile salt export
pump; I-BABP = intestinal bile acid–binding protein; FFA: free fatty
acid; TG: triglyceride; VLDL: very low density lipoprotein; RXR:
retinoid X receptor; LXR: liver X receptor; FXR: farnesoid-activated
receptor; SREBP: sterol response element–binding protein.

- Bays
H, Shepherd J. Diabetes, metabolic syndrome and dyslipidemia. In:
Management Strategies in Diabetes. Hackensack, NJ: Cambridge Medical
Publications, 2004:1-28.
- Bays
HE, Stein EA. Pharmacotherapy for dyslipidemia: current therapies and
future agents. Expert Opin Pharmacother 2003;4:1901-1938.
|
|


Myalgia is defined as muscle aches with or without muscle enzyme
elevation. Myopathy is often defined as creatine kinase levels >10
times the upper limit of normal associated with symptoms (myalgias).
Rhabdomyolysis may occur for many reasons, such as alcoholism (with
tremors), infection, trauma, marked increase in extraordinary exercise
(particularly without adequate hydration), seizures, genetic metabolic
abnormalities. Its diagnosis is somewhat subjective, but it is
typically characterized by creatine kinase levels >5 times the upper
limit of normal, elevations in other muscle enzymes, myoglobinuria,
hyperkalemia, hypocalcemia, hyperphosphatemia, hyperuricemia, metabolic
acidosis, and possibly renal insufficiency or renal failure. In
drug-induced rhabdomyolysis, symptoms of muscle weakness may be present
only 50% of the time.
Statin administration alone may cause myalgias and myopathy.
Statin monotherapy has even been described to result in rhabdomyolysis
in rare cases. One of the main concerns with the combined use of
statins with fibrates is the increased risk for myopathy and
rhabdomyolysis, particularly when gemfibrozil is the fibrate used.

- Bays
HE, Stein EA. Pharmacotherapy for dyslipidemia: current therapies and
future agents. Expert Opin Pharmacother 2003;4:1901-1938.
- Ballantyne
CM, Corsini A, Davidson MH, et al. Risk for myopathy with statin
therapy in high-risk patients. Arch Intern Med 2003;163:553-564.
- Bays
H. Existing and investigational combination drug therapy for
high-density lipoprotein cholesterol. Am J Cardiol 2002;90:30K-43K.
|
|


The use of statins and TZDs is common, given that (1) diabetes mellitus
is considered a CHD risk equivalent, (2) aggressive LDL-C level
lowering is recommended in patients with diabetes mellitus, (3) statins
and TZDs are common lipid-altering and antidiabetes agents,
respectively, and (4) dyslipidemia and diabetes mellitus often occur in
the same patient. One of the most active areas of clinical research for
metabolic syndrome and type 2 diabetes mellitus is the development of
pharmaceutical agents that possess both PPAR-α and PPAR-γ effects (dual
PPAR-α/γ agonists). Another area of research involves development of
agents that may affect other nuclear receptors.

- Bays
HE. Atherogenic dyslipidaemia in type 2 diabetes and metabolic
syndrome: current and possible future treatment options. British
Journal of Diabetes and Vascular Disease 2003;3:356-360.
- Ginsberg HN. Treatment for patients with the metabolic syndrome. Am J Cardiol 2003;91:29E-39E.
|
|


Commercial fish oil preparations are available that contain more than
1000 mg per capsule of omega-3 fatty acids, including eicosapentaenoic
acid (EPA) and docosahexaenoic acid (DHA). In general, most studies
have described benefits on blood triglyceride levels with combined
doses of EPA and DHA of at least 4 g, up to 9 g/day. Side effects of
fish oils include fishy aftertaste and dyspepsia, which may be improved
by refrigeration of agent prior to administration. Reports have
inconsistently noted an early, short-lived potential increase in blood
glucose levels in patients with diabetes mellitus. Fish oils may impair
platelet aggregation and increase bleeding time, potentially reducing
the risk for thrombosis. Clinical trials have not demonstrated
significantly increased risk of bleeding if used concomitantly with
anticoagulants (aspirin or warfarin).

- Kris-Etherton
PM, Harris WS, Appel LJ, et al. Fish consumption, fish oil, omega-3
fatty acids, and cardiovascular disease. Circulation 2002;106:2747-2757.
- Bays
HE, Stein EA. Pharmacotherapy for dyslipidemia: current therapies and
future agents. Expert Opin Pharmacother 2003;4:1901-1938.
|
|


The two major sources of circulating cholesterol are (1) synthesis
(from peripheral tissues and liver) and (2) absorption (from the
intestine). Once synthesized or absorbed, free cholesterol is
esterified. Depending upon the location or pathway, cholesteryl ester
may then be packaged into apolipoprotein (apo) B–containing
lipoproteins. Preferential delivery of cholesterol from peripheral
tissues (such as endothelial macrophages associated with arterial
cholesterol plaques) to the liver may occur with increased HDL
metabolic flux to the liver, which may reduce atherosclerotic plaques.
Elevated levels of HDL-C have been associated with a reduced risk for
CHD, which may occur by at least three mechanisms: (1) promotion of
peripheral cholesterol transport, (2) antioxidant/anti-inflammatory
effects, and (3) antithrombotic effects.

- Bays
H. Existing and investigational combination drug therapy for
high-density lipoprotein cholesterol. Am J Cardiol 2002;90:30K-43K.
- Bays H. Ezetimibe. Expert Opin Investig Drugs 2002;11:1587-1604.
|
|


Statins and niacin have different mechanisms of action and different
favorable effects on lipoprotein levels. Thus, the combined use of
statins and niacin may have complementary benefits on multiple
lipoprotein parameters.

- McKenney
JM, McCormick LS, Schaefer EJ, et al. Effects of niacin and
atorvastatin on lipoprotein subclasses in patients with atherogenic
dyslipidemia. Am J Cardiol 2001;88:270-274.
- Bays
H. Existing and investigational combination drug therapy for
high-density lipoprotein cholesterol. Am J Cardiol 2002;90:30K-43K.
|
|


The ADvicor Versus Other Cholesterol-modulating Agents Trial Evaluation
(ADVOCATE) was designed to compare the efficacy of a niacin
extended-release (ER)/lovastatin preparation with that of 2 common
statin monotherapies, atorvastatin and simvastatin. After 8 weeks'
administration at their starting doses, both niacin ER/lovastatin
1,000/40 mg and atorvastatin 10 mg lowered mean LDL-C levels by 38%.
After 12 weeks, niacin ER/lovastatin 1,000/40 mg lowered LDL-C levels
by 42%, while the 20-mg starting dose of simvastatin lowered LDL-C
levels by 35% (p <0.001). Additionally, niacin ER/lovastatin
increased HDL-C levels significantly more than either atorvastatin or
simvastatin at all compared doses (p <0.001), and provided
significant improvements in other lipid parameters such as
triglyceride, lipoprotein(a), apo A-I, and apo B levels. Equal or
greater reductions in LDL-C levels with greater, more multidimensional
improvement in other lipid parameters using combination niacin plus
statin therapy reduces CHD lipid risk factors more than the use of
either agent alone.

- Bays
HE, Dujovne CA, McGovern ME, et al. Comparison of once-daily, niacin
extended-release/lovastatin with standard doses of atorvastatin and
simvastatin (the ADvicor Versus Other Cholesterol-Modulating Agents
Trial Evaluation [ADVOCATE]). Am J Cardiol 2003;91:667-672.
|
|


This table describes relative terminology among the various
commercially available lipoprotein subclass analyses, and is not meant
to represent direct comparisons of the various methodologies with
respect to lipoprotein particle size and subclass distribution. In
general, a decrease in LDL and HDL particle size is most associated
with increased CHD risk. The implications of VLDL particle size are
less clear. As assessed by nuclear magnetic resonance, larger VLDL
particles are thought to be associated with higher CHD risk.
Conversely, it has been suggested that with Vertical Auto Profile, it
is the small remnant VLDL particles that are stronger predictors of CHD
risk than large, buoyant VLDL particles. The slide highlights the
complexity of comparing methods that differ substantially in how
lipoproteins are measured. There is no federal agency that regulates
the accuracy of the measurements, and obviously, there is no set
standard as to how results of lipoprotein particle size and subclass
distribution are reported.

- Bays
HE, McGovern ME. Once-daily niacin extended release/lovastatin
combination tablet has more favorable effects on lipoprotein particle
size and subclass distribution than atorvastatin and simvastatin. Prev
Cardiol 2003;6:179-188.
|
|


In a further review of the ADVOCATE data with a focus on relative
effects on lipoprotein subclass distribution, at all doses tested:
- Niacin ER/lovastatin was more effective than either atorvastatin or simvastatin in increasing LDL peak particle diameter.
- Niacin ER/lovastatin was more effective than either
atorvastatin or simvastatin in increasing the proportion of HDL in
"cardioprotective" 2b subclass (according to segmented gel
electrophoresis).

- Bays
HE, McGovern ME. Once-daily niacin extended-release/lovastatin
combination tablet has more favorable effects on lipoprotein particle
size and subclass distribution than atorvastatin and simvastatin. Prev
Cardiol 2003;6:179-188.
|
|


In a further review of the ADVOCATE data with a focus on relative
effects on lipoprotein subclass distribution, at all doses tested:
- Niacin ER/lovastatin was more effective than either
atorvastatin or simvastastin in reducing the proportion of LDL in
atherogenic small, dense IIIa/IIIb subclasses.
- Niacin ER/lovastatin was more effective than either atorvastatin or simvastatin in reducing LDL pattern B prevalence.

- Bays
HE, McGovern ME. Once-daily niacin extended-release/lovastatin
combination tablet has more favorable effects on lipoprotein particle
size and subclass distribution compared to atorvastatin and
simvastatin. Prev Cardiol 2003;6:179-188.
|
|


Isolated case reports have suggested an increased risk for liver and
muscle toxicity with previous niacin preparations used in combination
with statins. However, the ADVOCATE trial, using niacin
extended-release [ER]/lovastatin in doses ranging from 500 mg/20 mg to
2000 mg/40 mg and atorvastatin or simvastatin monotherapy 10–40 mg,
demonstrated that:
- 10 of 157 patients (6%) treated with niacin ER/lovastatin withdrew because of flushing
- Increases in mean alanine aminotransferase (ALT) levels were
significantly greater at all doses of atorvastatin and simvastatin
compared with niacin ER/lovastatin (although the mean ALT levels did
not rise above the upper limit of normal in any treatment group)
- No drug-induced cases of myopathy were observed
- No significant differences were seen in the incidence of
rash, hyperglycemia, hyperuricemia, or gastrointestinal complaints
between treatment groups.
The risk for flushing with niacin can be reduced with the use
of longer acting niacin preparations such as the ER preparation used in
this trial, as well as preventive measures such as avoiding spicy or
hot foods and drinks, avoiding alcohol while taking niacin, taking
aspirin or a nonsteroidal anti-inflammatory drug 30 minutes before
niacin, and administration of a small snack with niacin administration.
The risk for increased liver toxicity when niacin ER is combined with
statins is low if the agents are properly administered and
appropriately monitored. An increased risk for myopathy with this
combination was not supported by this trial. In fact, there is little
evidence from clinical trials that the use of moderate doses of niacin
increases muscle toxicity of any kind. However, myalgias and myopathy
may occur with statins alone. Finally, although not observed in this
trial, other adverse effects such as rash, hyperglycemia,
hyperuricemia, and gastrointestinal complaints may occur with niacin
and should be appropriately monitored.

- Bays
HE, Dujovne CA, McGovern ME, et al. Comparison of once-daily, niacin
extended-release/lovastatin with standard doses of atorvastatin and
simvastatin (the ADvicor Versus Other Cholesterol-Modulating Agents
Trial Evaluation [ADVOCATE]). Am J Cardiol 2003;91:667-672.
- Zhao
XQ, Morse JS, Dowdy AA, et al. Safety and tolerability of simvastatin
plus niacin in patients with coronary artery disease and low
high-density lipoprotein cholesterol (The HDL Atherosclerosis Treatment
Study). Am J Cardiol 2004;93:307-312.
- Bays
H. Commentary: Trial finds that simvastatin plus niacin is safe in
people with coronary artery disease and low HDL cholesterol.
Evidence-based Cardiovascular Medicine 2004;8:173-176.
- Bays
HE. Extended-release niacin/lovastatin: the first combination product
for dyslipidemia. Expert Rev Cardiovasc Ther 2004;2:485-501.
|
|


A study that has examined the potential CHD outcomes benefit of niacin
plus statin combination therapy was the HDL-Atherosclerosis Treatment
Study (HATS). In this placebo-controlled secondary prevention study,
160 patients with CHD, low HDL-C (average, 31 mg/dL), and "normal"
serum LDL-C levels (average, 125 mg/dL) were administered either niacin
(N; slow-release or immediate-release, mean dose 2.4 g/d) plus
simvastatin (S; mean dose 13 mg/d) or placebo with or without
antioxidant vitamins (AV) for 3 years. In the group receiving niacin
plus simvastatin without antioxidants, LDL-C levels were lowered by
42%; the LDL-C levels in the placebo groups were unaltered. HDL-C was
increased by 26% in the niacin plus simvastatin group. The combination
of niacin and simvastatin reduced CHD events by 60–90%, with about a
90% reduction seen in those subjects who did not take antioxidants,
possibly because the treatment-induced increase in HDL particle size
was blunted by antioxidants.
HATS was a small trial that clearly needs to be repeated in
order to verify the essential "cure" of atherosclerotic CHD with
combination niacin plus statin therapy. However, this trial does give
hope that the use of combination lipid-altering drugs with
complementary actions may not only provide superior effects on multiple
lipid parameters as compared with either agent alone, but may also
improve CHD outcomes.
Further support for the clinical benefit of niacin in
combination with statins was provided by Arterial Biology for the
Investigation of the Treatment Effects of Reducing Cholesterol
(ARBITER) 2, a 12-month study of 167 statin-treated patients with known
CHD and low HDL-C levels (<45 mg/dL) who were also administered
extended-release niacin or placebo. Patients receiving statin alone had
significant progression in atherosclerosis measured by carotid
intima–media thickness (p <0.001) while a nonsignificant tendency
toward progression (p=0.23) occurred in the statin-plus-niacin group.
Although the study was not powered to show differences in
cardiovascular event endpoints, there was a trend towards greater
reduction in clinical cardiovascular events in the statin-plus-niacin
patients (3.8%, versus 9.6% in the statin-only group; p=0.20).

- Brown
BG, Zhao XQ, Chait A, et al. Simvastatin and niacin, antioxidant
vitamins, or the combination for the prevention of coronary disease. N
Engl J Med 2001;345:1583-1592.
- Taylor
AJ, Sullenberger LE, Lee HJ, et al. Arterial Biology for the
Investigation of the Treatment Effects of Reducing Cholesterol
(ARBITER) 2: a double-blind, placebo-controlled study of
extended-release niacin on atherosclerosis progression in secondary
prevention patients treated with statins.Circulation 2004;110:3512-3517.
|
|


Multiple clinical trials of statins have demonstrated significant
reductions in CHD events compared to placebo. However, overall, the
risk of CHD compared to placebo has "only" been reduced on the order of
about 1/4 to 1/3. Thus, in clinical trials of statin monotherapy, the
majority of CHD events were not prevented. These data have prompted
studies of more aggressive lowering of LDL-C levels, which usually
requires combining other lipid-altering drugs with statins. In
addition, because of intriguing and provocative outcomes data (such as
those described with small CHD outcome studies of statins and niacin),
current studies are evaluating other investigational agents in
combination with statins that may provide complementary actions beyond
LDL-C lowering alone.
Abbreviations: AFCAPS/TexCAPS = Air Force/Texas Coronary
Atherosclerosis Prevention Study; ASCOT-LLA = Anglo-Scandinavian
Cardiac Outcomes Trial—Lipid-Lowering Arm; CARE = Cholesterol and
Recurrent Events; HPS = Heart Protection Study; LIPID = Long-Term
Intervention with Pravastatin in Ischaemic Disease; PROSPER =
Prospective Study of Pravastatin in the Elderly at Risk; 4S =
Scandinavian Simvastatin Survival Study; WOSCOPS = West of Scotland
Coronary Prevention Study.

- Bays
HE. Extended-release niacin/lovastatin: the first combination product
for dyslipidemia. Expert Rev Cardiovasc Ther 2004;2:485-501.
- Scandinavian
Simvastatin Survival Study Group. Randomised trial of cholesterol
lowering in 4444 patients with coronary heart disease: the Scandinavian
Simvastatin Survival Study (4S). Lancet 1994;344:1383-1389.
- Shepherd
J, Cobbe SM, Ford I, et al., for the West of Scotland Coronary
Prevention Study Group. Prevention of coronary heart disease with
pravastatin in men with hypercholesterolemia. N Engl J Med
1995;333:1301-1307.
- Sacks
FM, Pfeffer MA, Moye LA, et al., for the Cholesterol and Recurrent
Events Trial Investigators. The effect of pravastatin on coronary
events after myocardial infarction in patients with average cholesterol
levels. N Engl J Med 1996;335:1001-1009.
- Downs
JR, Clearfield M, Weis S, et al., for the AFCAPS/TexCAPS Research
Group. Primary prevention of acute coronary events with lovastatin in
men and women with average cholesterol levels: results of
AFCAPS/TexCAPS. JAMA 1998;279:1615-1622.
- Long-Term
Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group.
Prevention of cardiovascular events and death with pravastatin in
patients with coronary heart disease and a broad range of initial
cholesterol levels. N Engl J Med 1998;339:1349-1357.
- Heart
Protection Study Collaborative Group. MRC/BHF Heart Protection Study of
cholesterol lowering with simvastatin in 20 536 high-risk individuals:
a randomised placebo-controlled trial. Lancet 2002;360:7-22.
- Shepherd
J, Blauw GJ, Murphy MB, et al., on behalf of the PROSPER study group.
Pravastatin in elderly individuals at risk of vascular disease
(PROSPER): a randomised controlled trial. Lancet 2002;360:1623-1630.
- Sever
PS, Dahlof B, Poulter NR, et al., for the ASCOT Investigators.
Prevention of coronary and stroke events with atorvastatin in
hypertensive patients who have average or lower-than-average
cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes
Trial—Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised
controlled trial. Lancet 2003;361:1149-1158.
|
|


In the process of cholesterol transport, apo B–containing VLDL
particles are released to the circulation from the liver, and through
metabolism by endothelial lipoprotein lipases (LPL) and hepatic lipases
(HL), are catabolized into LDL particles. Once these LDL particles have
traversed arterial endothelial walls (along with their associated
cholesterol), the particles may become modified and undergo uptake by
subendothelial macrophages which express specialized or scavenger
receptors (SR). The result is the formation of cholesterol-laden
macrophages (otherwise known as foam cells) that may produce growth
factors (that may promote cell proliferation) and metalloproteinases
(that may promote cell matrix degradation)—all resulting in promotion
of the vulnerable atherosclerotic plaque that leads to CHD events.
In contrast to simply becoming engorged with cholesterol,
arterial-associated macrophages may also interact with HDL particles,
and through ABCA1, transport cholesterol to nascent HDL particles. Once
in the circulation, apo A-I–associated HDL particles may then be
metabolized through uptake at the liver by:
• Scavenger receptors, such as SR B1, that extract cholesterol
from HDL particles and then release HDL back into the circulation
• Putative receptors that mediate hepatic uptake and the subsequent catabolism of the entire HDL particle
This transport of cholesterol from peripheral
arterial–associated macrophages to the liver (peripheral cholesterol
transport) is thought to be an important process in reducing
atherosclerotic progression. CETP facilitates the exchange of
cholesterol from antiatherogenic apo A-I–containing HDL particles to
the atherogenic apo B–containing VLDL and LDL particles. Theoretically,
inhibition of CETP, in effect, breaks the bridge between the
antiatherogenic and atherogenic pathways, leading to a greater
redirection of cholesterol back to the liver for clearance and away
from the atherogenic pathway.

- Brewer
HB Jr, Santamarina-Fojo S. Clinical significance of high-density
lipoproteins and the development of atherosclerosis: focus on the role
of the adenosine triphosphate–binding cassette protein A1 transporter.
Am J Cardiol 2003;92:10K-16K.
- Bays
HE, Stein EA. Pharmacotherapy for dyslipidemia: current therapies and
future agents. Expert Opin Pharmacother 2003;4:1901-1938.
- Bays
HE. Extended-release niacin/lovastatin: the first combination product
for dyslipidemia. Expert Rev Cardiovasc Ther 2004;2:485-501.
|
|


In addition to effects upon absolute blood lipid levels, CETP also has
a role in promoting abnormalities in lipoprotein particle size and
subclass distribution. Adiposity, genetic predisposition, and sedentary
lifestyle promote dysfunctional adipose tissue (adiposopathy).
Adiposopathy is associated with abnormalities in the release of
hormones, cytokines, enzymes, molecules, and other factors from fat
cells that may contribute to an array of metabolic abnormalities
including dyslipidemia, insulin resistance, and hypertension. For
example, the release of free fatty acids from dysfunctional fat cells
may contribute to fatty liver and fasting hypertriglyceridemia with
increased VLDL particles. CETP facilitates the exchange of triglyceride
(for cholesterol) from VLDL particles to HDL particles, which are more
readily cleared by the kidneys resulting in lower HDL-C levels. CETP
may also facilitate the exchange of triglyceride (for cholesterol) from
VLDL particles to LDL particles. The more triglyceride-rich LDL
particles undergo metabolism by various lipases resulting in small,
dense LDL particles. Thus, CETP contributes to the common lipid profile
characterized as "metabolic syndrome," which often includes fatty
liver, fasting hypertriglyceridemia, low HDL-C levels, and small, dense
LDL particles. This figure does not depict the important contribution
of postprandial hypertriglyceridemia, which often occurs in these same
patients, wherein elevated postprandial chylomicrons (from the
intestine) may also contribute to hypertriglyceridemia, the atherogenic
lipid profile described above, and the creation of chylomicron remnant
particles, which may be significantly atherogenic.

- Bays
HE. Extended-release niacin/lovastatin: the first combination product
for dyslipidemia. Expert Rev Cardiovasc Ther 2004;2:485-501.
- Bays HE. Current and investigational antiobesity agents and obesity therapeutic treatment targets. Obes Res 2004;12:1197-1211.
- Bays
H, Abate N, Chandalia M. Adiposopathy: sick fat causes high blood
sugar, high blood pressure and dyslipidemia. Future Cardiology
(2005) 1(1), 39-59.
|
|


Inhibiting CETP may improve lipoprotein particle size and subclass
distribution. For example, centenarians ("probands") and their
offspring have been suggested to have larger HDL and LDL particle size
(as well as lower prevalence of hypertension, cardiovascular disease,
and the metabolic syndrome), which may, at least in part, be due to the
presence of a CETP mutation associated with decreased CETP activity.
Thus, CETP inhibition may not only improve lipid blood levels, but may
also improve HDL and LDL particle sizes. Therefore, inhibiting CETP may
be an attractive treatment target for the purpose of CHD risk
reduction.

- Barzilai
N, Atzmon G, Schechter C, et al. Unique lipoprotein phenotype and
genotype associated with exceptional longevity. JAMA 2003;290:2030-2040.
|
|


Torcetrapib is a CETP inhibitor that has been shown in monotherapy to
result in a dose-dependent increase in HDL-C (15–70%), as well as a
modest decrease in LDL-C (up to ~20%) and inconsistent effect on
triglyceride (TG) levels.

- Clark
RW, Sutfin TA, Ruggeri RB, et al. Raising high-density lipoprotein in
humans through inhibition of cholesteryl ester transfer protein: an
initial multidose study of torcetrapib. Arterioscler Thromb Vasc Biol
2004;24:490-497.
|
|


According to other preliminary data released by the manufacturer
(Pfizer), torcetrapib monotherapy has been shown in an 8-week phase II
trial to increase HDL-C levels by 55% and lower LDL-C levels by 20%.
Across the dose range of atorvastatin (10–80 mg), torcetrapib 90 mg in
combination with atorvastatin has the potential to raise HDL-C levels
by 50% and lower LDL-C levels by 70–80%. The addition of torcetrapib 90
mg to atorvastatin 20 mg resulted in an additional LDL-C lowering of
15% over atorvastatin alone, which was comparable to the 20% LDL-C
lowering found with torcetrapib 120 mg monotherapy.
Torcetrapib is not currently being planned as a monotherapy
drug. However, a combination product is in phase III development,
including CHD outcomes studies. In one example, the effects of
torcetrapib/atorvastatin combination on atherosclerotic progression is
being evaluated by intravascular ultrasound in patients with
nonobstructive CHD (20–50% stenosis).

- Bays
HE, Stein EA. Pharmacotherapy for dyslipidemia: current therapies and
future agents. Expert Opin Pharmacother 2003;4:1901-1938.
|
|


Statins are currently the most prescribed lipid-altering drugs because
of their efficacy in favorably altering blood lipid levels, safety and
tolerability, and proven benefits on reducing atherosclerotic CHD
events. Combination lipid-altering drug therapy with statins is often
indicated for patients: (1) who are unable to achieve recommended
treatment goals with statin monotherapy, (2) who may be at risk for
intolerance, toxicity, or adverse drug interactions with higher-dose
statin monotherapy, or (3) who may benefit from the use of one or more
lipid-altering drugs in combination with statins resulting in
complementary benefits towards further reduction in CHD risk. Some of
the lipid-altering agents most commonly used in combination with
statins include ezetimibe, bile acid sequestrants, PPAR agonists, fish
oils, and niacin. A potentially important investigational
lipid-altering drug combination is statin in combination with a
cholesteryl ester transport inhibitor.
|
|
|

















































